This is the last article in a special five-part Forkast.News series on blockchain, Covid-19 and the future of healthcare. To learn more about this series, please see the full episode list here.
——————————————————————————
Global regulatory bodies have been approving Covid-19 vaccines at a cracking pace, with three vaccines now approved in the West. But this green light is just the beginning. In the months ahead, Pfizer/BioNTech, Moderna and Oxford/AstraZeneca need to scale up their production lines, fortify their supply and distribution chains.
While the pharmaceutical companies and vaccine manufacturers are working nonstop to manufacture and certify millions of vaccine vials, the global freight industry is using all available intelligence to prepare for the unique and unprecedented challenge of delivering vaccines to the world’s 7.8 billion people.
Regulatory bodies are also being charged with the heavy task of developing equitable and ethical schemes to deliver billions of doses around the world.
The delivery of Covid-19 vaccines is as critical as the discovery. If done properly, leveraging blockchain technology like never before, this momentous task could make history.
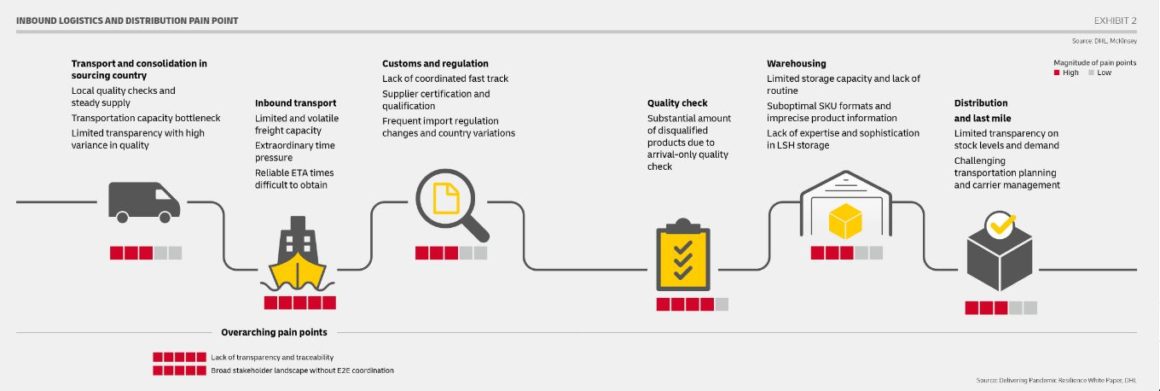
Getting a vaccine safely from point of manufacture to the recipient involves overcoming a multitude of hurdles when it comes to logistics, storage, packing facilities and regulations. Vaccines are highly sensitive to excessive vibration and temperature changes, requiring specialized refrigerators, ultra-low temperature (ULT) freezers, plus large and specialized transport vehicles. So it’s no surprise that roll-out teething problems have arisen that are causing slower than expected vaccination deliveries and inoculation, along with vaccine waste.
Roll-out teething problems
Currently, the supply of raw materials and perfect alignment of critical consumables is a significant issue — the synchronization of temperature control, inventory and status of vials, consumables, GPS tracking and tracing among other things.
Blockchain and distributed ledger technology has an essential part to play ensuring efficient vaccine distribution, as it can integrate real-time tracking capabilities with updates on a myriad of parameters.
In Japan, a vaccine roll-out was scheduled to start yesterday, but a shortage of unused syringes may instead lead to wastage of millions of Pfizer/BioNTech vaccine doses at a time when the country is battling its third and most serious wave of Covid-19 infections. According to Japan’s Health Minister, standard syringes in use in Japan are unable to extract the sixth and final dose from each vial manufactured by the U.S. drug-maker. Such an oversight is bound to frustrate the country’s inoculation program, particularly as it readies itself for the re-scheduled 2021 Olympics.
In stark contrast, an exciting example is the apparent success of the Israeli vaccination campaign, which has seen the number of infections fall significantly, according to preliminary analysis reported by the Weizmann Institute of Science. Strongly correlated with the digitized healthcare system in Israel, pharmaceutical companies not only receive data quickly and reliably, but importantly they get significantly more data than from traditional studies. In the 65-plus age group, there were 56% fewer infections, 42% fewer hospitalizations and 35% fewer Covid-19 deaths following the second dose.
Vaccine waste: an unaffordable problem
The sheer scale and concurrent demand of the Covid-19 vaccination programs worldwide present a unique challenge. The vaccines need to reach every country in every continent and every person age 16 and over. Here again, blockchain could be an ideal tool, as the technology can be scalable and able to store high quantities of data, verifiable in real-time. The approved vaccines require at least one to two shots per person, so factoring in the typical 20%-30% loss during transit and storage, the requirement could top 20 billion doses.
Tracking and tracing millions of different components is crucial in calculating vaccine wastage rate — a key input to anticipate demand. With around 20 billion units of vaccine potentially required globally, accurate estimates of wastage at every stage of the supply chain are fundamental. This is particularly pertinent to poorer and more remote communities.
UNICEF and Gavi Alliance, both with decades of experience running immunization programs, show that historically the vaccine supply chain and delivery can take years to stabilize, depending on the geography. For example, the Pulse Polio immunization scheme in India took more than a decade to cover 100% of the population’s children.

Gavi, in alliance with the World Health Organization (WHO) Covax program, is a global scheme to vaccinate people in poor and middle income countries around the world against the coronavirus. It aims to deliver at least two billion Covid-19 vaccine doses by the end of 2021 to cover 20% of the most vulnerable people in 91 such countries, mostly in Africa, Asia and Latin America.
The pharmaceutical industry historically has an average spoilage rate of between 4% and 12% for each shipment, costing companies approximately US$34.1 billion in 2019.
Ramping up air freight: certification, billion-dollar infrastructure required
Few airlines have the certifications — “CEIV Pharma,” or the Centre of Excellence for Independent Validators in Pharmaceutical Logistics, granted by the International Air Transport Association (IATA) — to transport vaccines. State-of-the-art infrastructure is also required, including ULT warehouses located strategically close to key air-hubs and specially-fitted aircraft to transport vaccine cargo.
In addition to the physical security and also infrastructure required to handle and deliver billions of vaccines, FedEx, UPS, DHL and other integrated logistics companies are also strengthening their “cold chain” plans and software systems to streamline this Herculean task.

FedEx recently launched SenseAware ID, their next-generation sensor-based proprietary technology. It provides enhanced package visibility for shipments using a compact sensor that transmits the location every two seconds. FedEx views this technology as a critical “safe feature” for the anticipated vaccine distribution efforts. FedEx has more than 90 cold chain facilities across the Americas, Asia, Australia and Europe and it plans to open additional facilities.
Etihad Cargo, the cargo and logistics arm of Etihad Aviation Group, strengthened its pharmaceutical logistics expertise with the launch of PharmaLife, a specialised pharma and healthcare product that replaces the carrier’s TempCheck product. Features of the platform include prioritized and highly trained ground handling and loading services personnel, rapid transit to temperature-controlled storage facilities, temperature-controlled cargo holds in the aircraft, and connections to over 83 global destinations.
Lufthansa Cargo has hubs with cold-storage facilities in Frankfurt, Munich and Chicago that can handle large amounts of pharmaceutical shipments. Their new product, Covid-19 Temp Premium, aims to provide seamless monitoring of temperature- and time-sensitive vaccine shipments, using aircraft that have been specifically fitted to safely and efficiently freight gigantic pallets of vaccines.
This orchestrated convergence and integration of blockchain, information management, predictive analytics, robotics and artificial intelligence (AI) will establish a more reliable, robust cold chain of custody for these most critical vaccines.
Constraints on vaccine delivery and distribution
The Moderna vaccine is stored and transported at a temperature of -20 degrees Celsius (-4 degrees Fahrenheit), then thawed, and dispensed individually by a licensed pharmacist. It can be stored at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit) for 30 days. These are temperatures found in normal freezers and refrigerators, which may give Moderna an edge because it makes mass distribution in cities easier, and less challenging for delivery to rural areas and low-income countries.
By contrast, the Pfizer/BioNTech vaccine must be shipped and stored at -70 to -80 degrees Celsius (-94 to -112 degrees Fahrenheit), a fact that has led global health officials to fear that it may be disproportionately accessible only to wealthy countries and communities that can afford ULT freezers. Through the WHO-backed Covax program, Pfizer has committed to supply up to 40 million doses of its Covid-19 vaccine to poor and middle-income countries.
Typically, because of the supportive infrastructure required to administer vaccinations, a visit to the local hospital outpatient section is needed, which is too centralized to be ideal in most communities and countries. To counter this issue, Pfizer has created a temperature-controlled container about the size of a small suitcase that can stored and shipped at -70 degrees Celsius, holding 1,000 to 5,000 vials for 10 days, before requiring more dry ice. But once thawed, the vaccine is only efficient for five days if refrigerated at usual temperatures of 2 to 8 degrees Celsius (or 35.6 to 46.4 degrees Fahrenheit). Another issue is that it comes in packs of 975 doses, which cannot yet be split into smaller batches, meaning it can be wasteful to send it to smaller hospitals or care homes with less than the number of inoculations.
The Oxford/AstraZeneca vaccine can be transported and stored at standard refrigeration temperatures for up to six months, which gives it a huge competitive benefit.
Booster compliance — availability, mix and match
A separate issue that will challenge all the available vaccines is compliance. All Covid-19 vaccines require a booster shot 21 to 28 days after the first in order to reach 95% protection. There are concerns that people will forget which vaccine they received, or completely forget to get their second jab.
As reported last week, surprising data has emerged that mixing and matching shots, plus delaying booster shots confers improved protection against Covid-19. Variant strains of Covid are a cause for concern and may thwart the entire project. However, the mRNA technology used in both the Pfizer/BioNTech, Moderna and Astra-Zeneca’s vaccine platforms can easily be adapted to target new strains, with all groups working on rapid “new boosters” as add-ons to the initial jab.
It will take multiple “Big Pharma” vaccines to overcome this pandemic. Delivering each and every shot to all of us, while managing to stay within the storage and handling environment parameters, and ensuring flawless integrity and minimal loss, can only be done efficiently with the use of blockchain. Blockchain offers an immutable, efficient system that will help all parties along the supply chain to verify that vaccines and vaccine supplies are manufactured, packed, transported, stored and administered properly.
It is indeed a most momentous task. But thanks to technology, there is a bright light at the end of the tunnel.